RESEARCH + DEVELOPMENT + INNOVATION
Clinical Studies
DiagnoX technology is backed by rigorous scientific research and clinical validation. Explore our peer-reviewed studies that demonstrate the safety, accuracy, and efficacy of our non-invasive glucose monitoring system.
RESEARCH + DEVELOPMENT + INNOVATION
Clinical Studies
DiagnoX technology is backed by rigorous scientific research and clinical validation. Explore our peer-reviewed studies that demonstrate the safety, accuracy, and efficacy of our non-invasive glucose monitoring system.
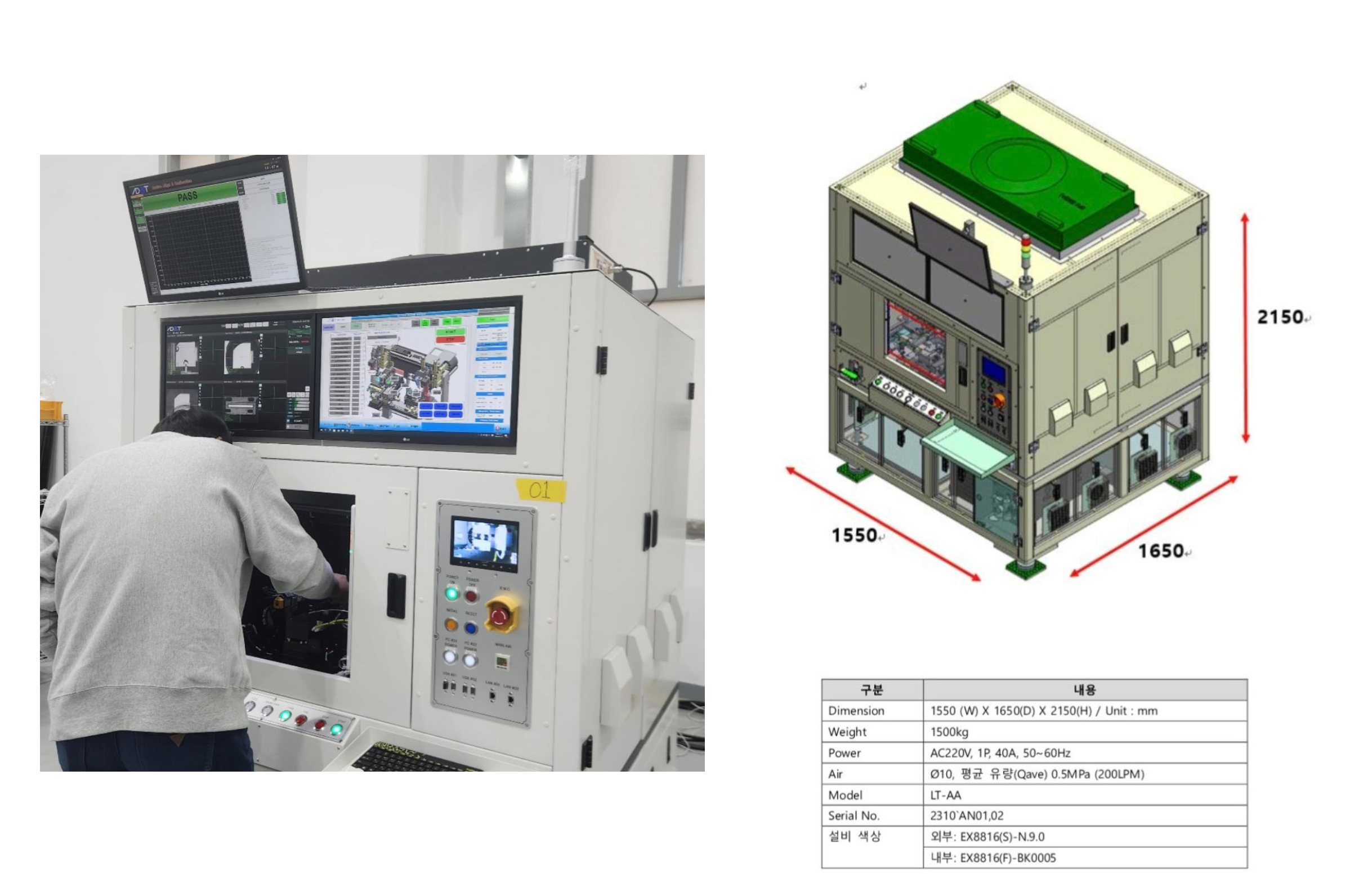
Product Working Flow
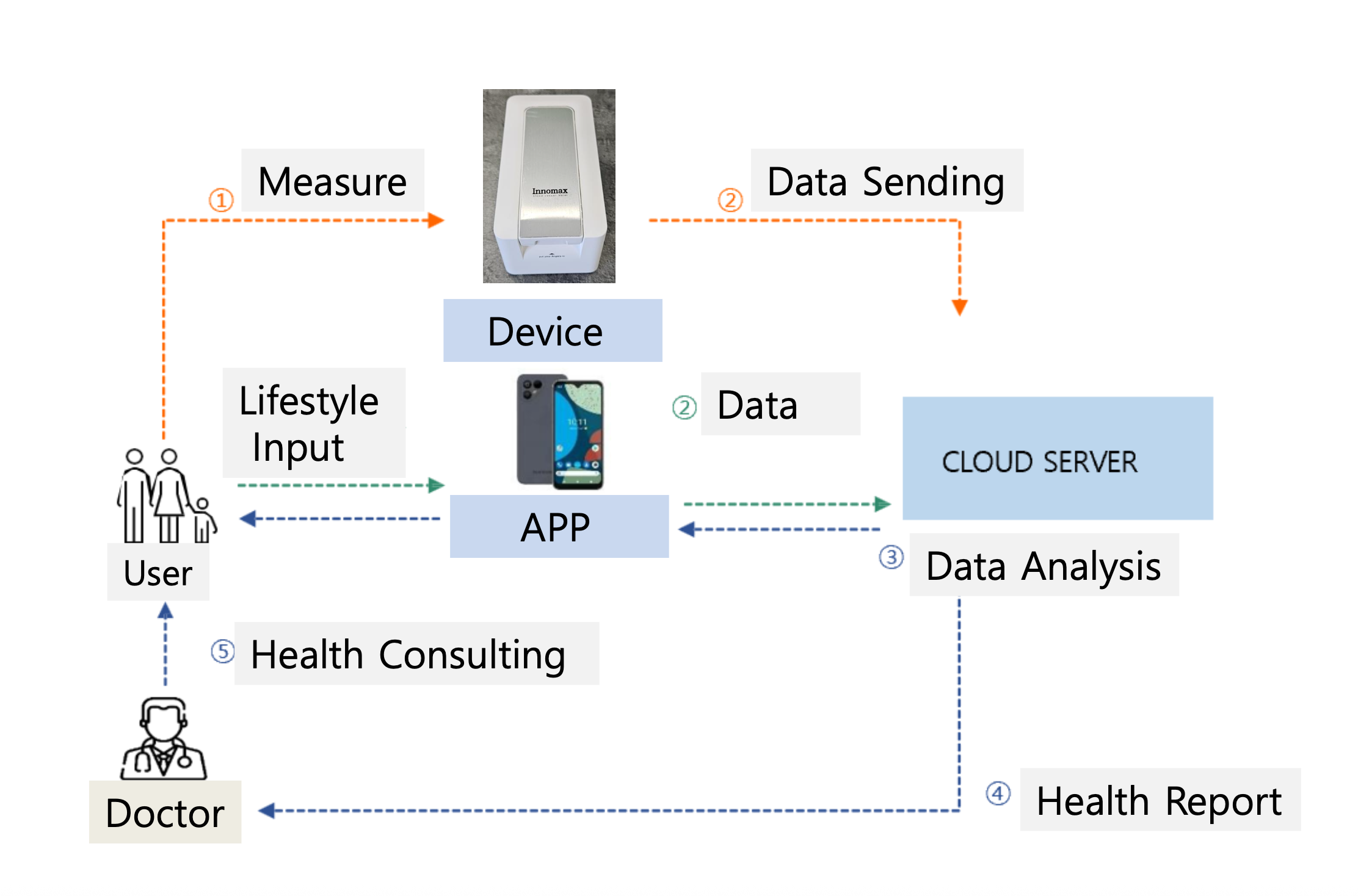
DiagnoX Technology
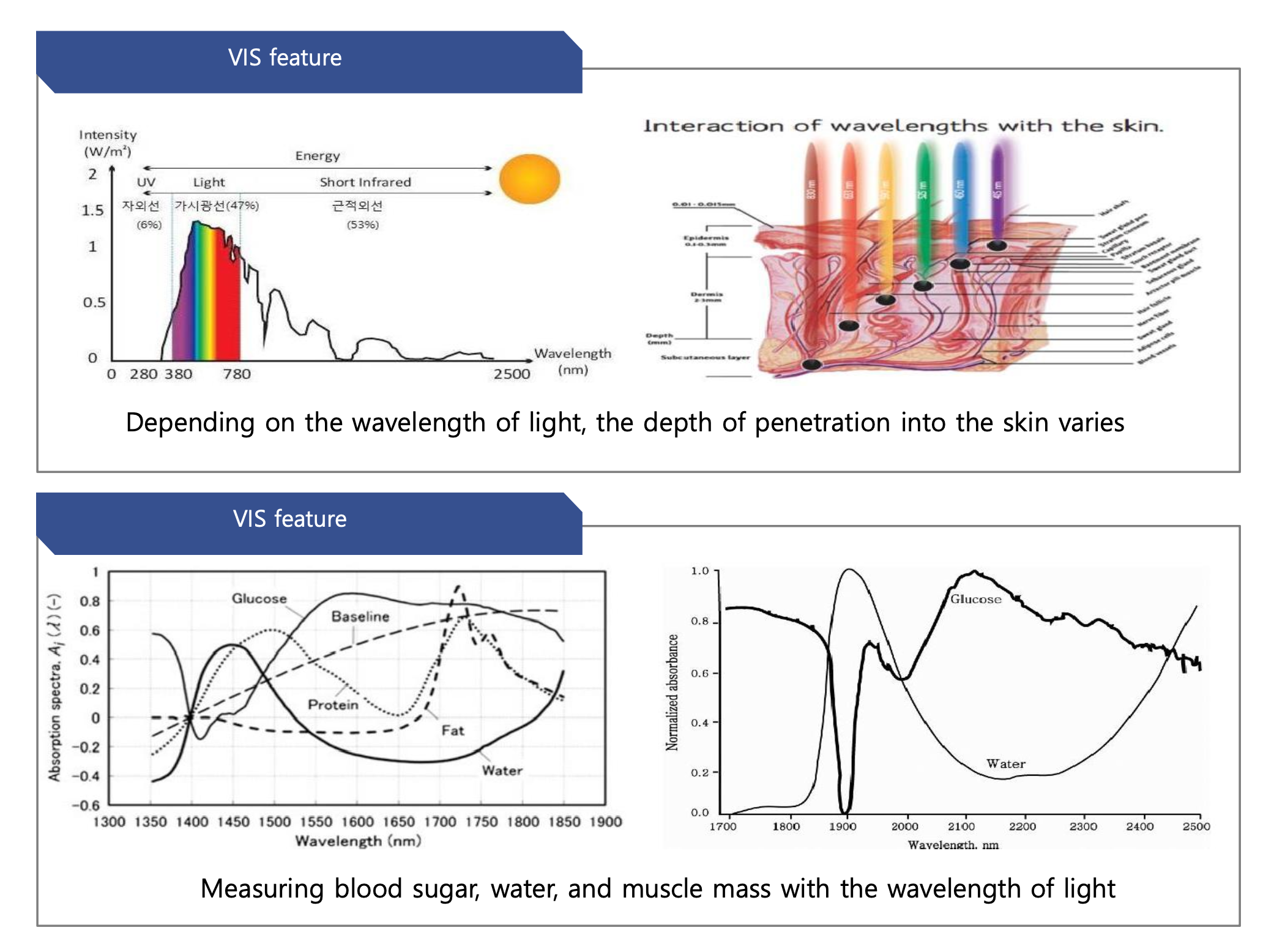
Clinic App
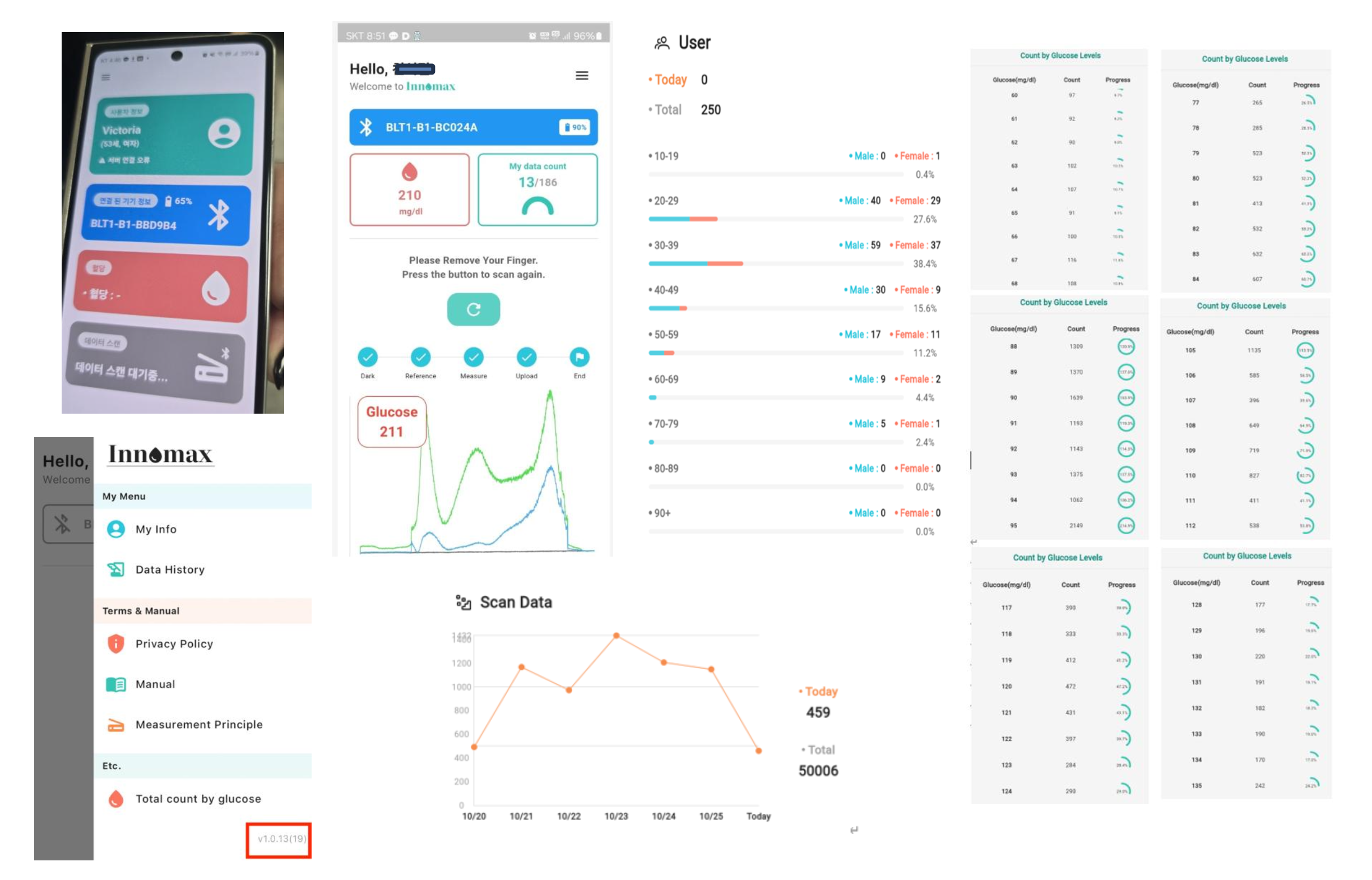
Factory Machine